Developability of Biotherapeutics Computational Approaches
Coordonnateurs : Kumar Sandeep, Kumar Singh Satish
Biopharmaceuticals are emerging as frontline medicines to combat several life-threatening and chronic diseases. However, such medicines are expensive to develop and produce on a commercial scale, contributing to rising healthcare costs. Developability of Biotherapeutics: Computational Approaches describes applications of computational and molecular modeling techniques that improve the overall process of discovery and development by removing empiricism.
The concept of developability involves making rational choices at the pre-clinical stages of biopharmaceutical drug development that could positively impact clinical outcomes. The book also addresses a general lack of awareness of the many different contributions that computation can make to biopharmaceutical drug development.
This informative and practical reference is a valuable resource for professionals engaged in industrial research and development, scientists working with regulatory agencies, and pharmacy, medicine, and life science students and educators. It focuses primarily on the developability of monoclonal antibody candidates, but the principles described can also be extended to other modalities such as recombinant proteins, fusion proteins, antibody drug conjugates and vaccines.
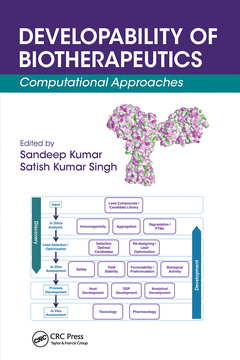
The book is organized into two sections. The first discusses principles and applications of computational approaches toward discovering and developing biopharmaceutical drugs. The second presents best practices in developability assessments of early-stage biopharmaceutical drug candidates.
In addition to raising awareness of the promise of computational research, this book also discusses solutions required to improve the success rate of translating biologic drug candidates into products available in the clinic. As such, it is a rich source of information on current principles and practices as well as a starting point for finding innovative applications of computation towards biopharmaceutical drug development.
PRINCIPLES OF BIOPHARMACEUTICAL INFORMATICS. Biopharmaceutical Informatics: Applications of Computation in Biologic Drug Development. Computational Methods in the Optimization of Biologic Modalities. Understanding, Predicting, and Mitigating the Impact of Posttranslational Physiochemical Modifications, Including Aggregation, on the Stability of Biopharmaceutical Drug Products. Preclinical Immunogenicity Risk Assessment of Biotherapeutics. Application of Mechanistic Pharmacokinetic–Pharmacodynamic Modeling toward the Development of Biologics. Challenges in High-Concentration Biopharmaceutical Drug Delivery: A Modeling Perspective. DEVELOPABILITY PRACTICES IN THE BIOPHARMACEUTICAL INDUSTRY. Best Practices in Assessment of Developability of Biopharmaceutical Candidates. Best Practices in Developability Assessments of Therapeutic Protein Candidates in the Biopharmaceutical Industry. Best Practices in Assessing the Developability of Biopharmaceutical Candidates. Developability Assessment Workflows to De-Risk Biopharmaceutical Development.
Sandeep Kumar, PhD, is a senior principal scientist at Pfizer, Inc. in the Biotherapeutics Pharmaceutical Sciences group, working on computational modeling and developability risk assessments of a wide variety of biotherapeutic drug candidates, including monoclonal antibodies, antibody-based therapeutics, fusion proteins, vaccines, and antibody–drug conjugates. He earned his PhD in computational biophysics from the Molecular Biophysics Unit of the Indian Institute of Science, Bangalore, India, and performed postdoctoral research on proteins at the National Cancer Institute in Frederick, Maryland, a part of the National Institutes of Health. He has been an assistant professor (research-track) at Georgetown University Medical Center, Washington, DC; an assistant professor at the Indian Institute of Technology, Kanpur; and an associate research scientist at Johns Hopkins University, Baltimore, Maryland. He has more than 17 years of experience in computational protein sciences. He has contributed more than 60 research papers, review articles, and book chapters, has delivered more than 40 invited talks, and has chaired sessions of several conferences. He has won several fellowships and awards, including a Fogarty visiting scientist fellowship at NIH and the 2014 Ebert Prize from the American Pharmacists Association. He has also mentored several graduate students and postdoctoral scientists.
Satish Kumar Singh, PhD, is a research fellow at Pfizer, Inc. in the Biotherapeutics Pharmaceutical Sciences group, and an adjunct professor of the Department of Physical Pharmaceutical Chemistry at Uppsala University in Sweden. His responsibilities include leading formulation, product, and process development activities for biologics and therapeutic vaccines. He earned his PhD in chemical engineering from Kansas State University. He has more than 25 years of industrial experience and has published more than 50 peer-reviewed articles with emphasis on the col
Date de parution : 06-2021
15.6x23.4 cm
Date de parution : 11-2015
Ouvrage de 297 p.
15.6x23.4 cm
Thème de Developability of Biotherapeutics :
Mots-clés :
Developability Assessment; T-cell Epitopes; assessment; Aggregation Prone Regions; aggregation; Aggregation Propensity; propensity; Met Oxidation; models; Asn Deamidation; protein; Immunogenicity Risk; coarse-grained; Biologic Drug; simulations; TMDD Model; neonatal; Ph Ar; receptor; CG Model; biologic; Ich Q8; CG Site; ADME Property; Clo Ne; PDB Entry; mAb1 Solutions; Physicochemical Degradation; MHC Class Ii; CG Simulation; Signal Peptide; Risk Assessment; Potential T-cell Epitopes; Elastic Network Model; Data Set


